Table of Content
Blog Summary:
A concise, practical guide examining the critical role of QA compliance in pharmaceutical app development and maintenance. The blog will address regulatory drivers, core quality assurance processes, documentation, digital validation, and challenges faced by US and global pharma teams in creating compliant, safe, and high-quality digital solutions for the industry.
Table of Content
Pharmaceutical software operates in a highly stringent digital ecosystem, and even a small flaw can cause life-altering consequences. Therefore, this software requires a higher level of QA compliance, whether facilitating drug traceability, managing clinical trial data, or supporting patient adherence.
Many important regulatory frameworks, such as EMA guidelines, 21 CFR Part 11, and GxP standards, make compliance and quality assurance necessary, rather than optional. The repercussions of non-compliance are plenty, from delayed product releases to hefty fines.
As the pharmaceutical industry extensively adopts digital transformation, QA teams must ensure that innovation doesn’t outpace inspection readiness. In this blog, we discuss the way to implement Quality Assurance and Compliance in every important phase of pharma app development.
Whether it’s clinical trials, drug development, patient engagement, or manufacturing, a pharmaceutical app has become central to several things. From being a supportive tool, this app has evolved into a major digital health infrastructure in recent years.
However, buggy pharmaceutical software can often malfunction during manufacturing control, lose clinical trial data, and even miscalculate dosages. It can lead to catastrophic outcomes, including regulatory penalties, product recalls, and legal action.
These apps must comply with stringent global regulations to overcome one of the biggest challenges of navigating the real-time delivery of physical drug products. The following are popular regulatory bodies:
These regulatory bodies must adhere to various frameworks, including GMP, 21 CFR Part 11, GAMP 5, and ICH Q10. All of these factors necessitate the validation of systems, ensuring traceability, and maintaining data integrity. These standards are necessary to provide full protection to patients from harm. It ensures the proper safety and therapeutic efficacy of public trust.
In today’s time, digital pharmaceutical tools reflect the same level of validation, rigor, and oversight as the medications they support. Hence, QA compliance is not only necessary for meeting audit requirements but also for preserving brand reputation and ensuring patient safety.
In this horizon, the quality of code keeps the same importance as the quality of chemistry, especially for mobile apps and cloud-based LIMS for drug delivery.
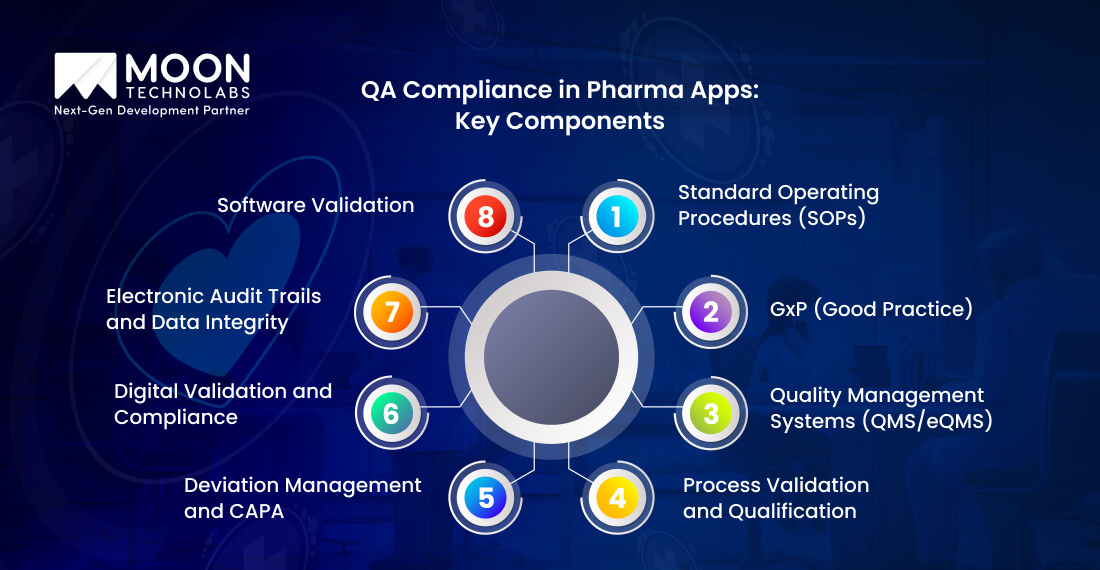
A structured framework is necessary for achieving quality assurance compliance in pharmaceutical apps. The framework also combines regulatory alignment with the top software practices.
The following are important components that are necessary for compliant pharma app development and maintenance;
In pharmaceutical apps, SOPs outline the procedures for every development and testing task. These tasks include requirement gathering, validation, post-release monitoring, and more. These are important documents that ensure traceability, consistency, and accountability across different teams and vendors.
Whether it’s GxP (Good Clinical Practice), GDP (Good Distribution Practice), or GMP (Good Manufacturing Practice), they are appropriate for digital systems. For instance, software used in a clinical trial should always follow GCP to ensure the accuracy of data capture and patient safety. Additionally, apps responsible for managing production must align precisely with GMP protocols for batch integrity and equipment control.
eQMS comes with an integrated platform for automation of compliance and quality assurance. These systems are useful to manage training records, SOPs, CAPA workflows, and change controls.
They accomplish this by maintaining audit-ready and secure documentation. Additionally, they provide comprehensive support for the approval workflow and version control.
A highly effective electronic QMS (eQMS) is necessary to ensure the entire compliance lifecycle aligns with regulations and role-based access. It centralizes everything, including audit trails, training records, document management, Corrective and Preventive Actions (CAPA), and change control.
Compliance relies on documentation that should be accurate, complete, and highly accessible. It includes validation protocols, requirement specs, deviation logs, test results, and more. Many features play a vital role in making documentation inspection-ready and traceable, which also satisfy the requirements of 21 CFR Part 11.
A rigorous and proper validation is essential for every pharmaceutical app. It reflects the performance of the app under various intended conditions, including operational qualification, installation qualification, and performance qualification. To ensure software is in a validated state during its lifecycle, pre-launch validations and post-deployment monitoring become necessary.
A formal CAPA process is necessary to address anomalies, bugs, or any deviations, ensuring safety for both continuous improvement and compliance. Hence, a QA team needs to analyze, trace, and resolve multiple root causes. They also need to document several corrective steps, which can range from a failed validation script to a post-release software defect.
Apart from functional testing, a modern pharmaceutical app business model requires data integrity, stringent validation, and documentation standards that are governed by regulators worldwide. A perfect digital tool adheres to QA compliance in pharmaceutical software throughout its lifecycle.
Pharmaceutical companies need to maintain tamper-proof audit trails in accordance with GAMP 5 and 21 CFR Part 11 guidelines. Pharma apps need to log every modification, user action, data transaction, etc. Tools that are useful for digital signatures, electronic batch records (EBRs), and secure immutable logs also work effectively in preserving data integrity, compliance, and traceability during regulatory checks.
System-level validation must reflect the ongoing qualification of the software and the supporting environment. It includes various elements such as requirement traceability, risk assessments, user acceptance testing (UAT), networks, hardware, and cloud platforms.
It also involves verifying platform configurations, infrastructure-as-a-service (IaaS), and third-party integrations. Hence, maintenance activities are necessary to demonstrate that the software is in a compliant state after launch. These are necessary to ensure everything aligns perfectly with GMP and GxP.
We help you achieve full compliance with your pharma software through access controls, secure data handling, and audit-ready validation processes, among other measures.
With the fast-paced digital transformation, evolving regulations, and global supply chain complexities, the pharma team faces huge pressure. It further creates several compliance challenges. Let’s explore some of these challenges and their solutions.
To keep up-to-date with the changing EMA, FDA, ICH, and global requirements. It’s indeed exhausting for teams that handle audits and documentation.
Solutions:
To overcome this challenge, consider utilizing regulatory monitoring teams. You need to integrate this tool into your eQMS to adapt and flag SOPs in real-time. Your team should also have a compliance calendar and regular internal audits.
The merger of new pharmaceutical apps with outdated or old infrastructure often creates challenges, such as inefficiencies and validation gaps.
Solution:
You can minimize the gap between the new and old systems by using middleware and also validation-friendly APIs. You can maintain documentation to reflect validated interoperability for quality assurance purposes.
Outsourcing often presents challenges, such as compliance risk, particularly when dealing with global vendors.
Solutions:
It’s advisable to define foolproof supplier qualification programs and implement third-party systems into your QA and compliance frameworks using GxP-compliant checklists.
Digital workflows maximize exposure to breaches, tampering, and unauthorized access.
Solutions:
You can implement 21 CFR Part 11-compliant audit trails, multi-factor authentication, encrypted backups, and routine cybersecurity validation as an important part of software lifecycle QA.
QA and compliance failures often take place due to a lack of awareness and insufficient training.
Solution:
To address this challenge, you can implement role-based training management systems with retraining alerts, automated tracking, and SOP testing within your electronic Quality Management System (eQMS).
Manual processes are insufficient to support the rapid product cycles.
Solutions:
You can harness the potential advantages of digital tools such as electronic documentation, automated testing, and cloud-based QMS. They help you scale QA compliance in pharmaceutical apps with higher efficiency.
We deliver complete and flawless performance, along with robust security, through our high-grade QA solutions. Our solutions are tailored to ensure the success of your app.

When it comes to maintaining quality assurance and compliance in pharmaceutical software, several key practices are essential. Let’s explore some of those that help you meet global regulatory expectations.
In the pharmaceutical industry, quality assurance compliance is not only a regulatory requirement but also a necessity to ensure patient safety. It’s also essential for building brand trust and maintaining product integrity.
Since pharmaceutical software has become pivotal for manufacturing and patient operations, implementing QA and compliance is necessary throughout the medicine delivery app development lifecycle. A digital-first and proactive approach encourages teams to stay ahead with the evolving regulations, with the assurance of product reliability.
Moon Technolabs has extensive experience in creating pharmaceutical software, ensuring its regulatory compliance. Our QA experts do everything to help you achieve inspection readiness with confidence, whether it’s end-to-end app testing, eQMS integration, or regulatory consulting.
01
02
03
04
Submitting the form below will ensure a prompt response from us.